What We Do
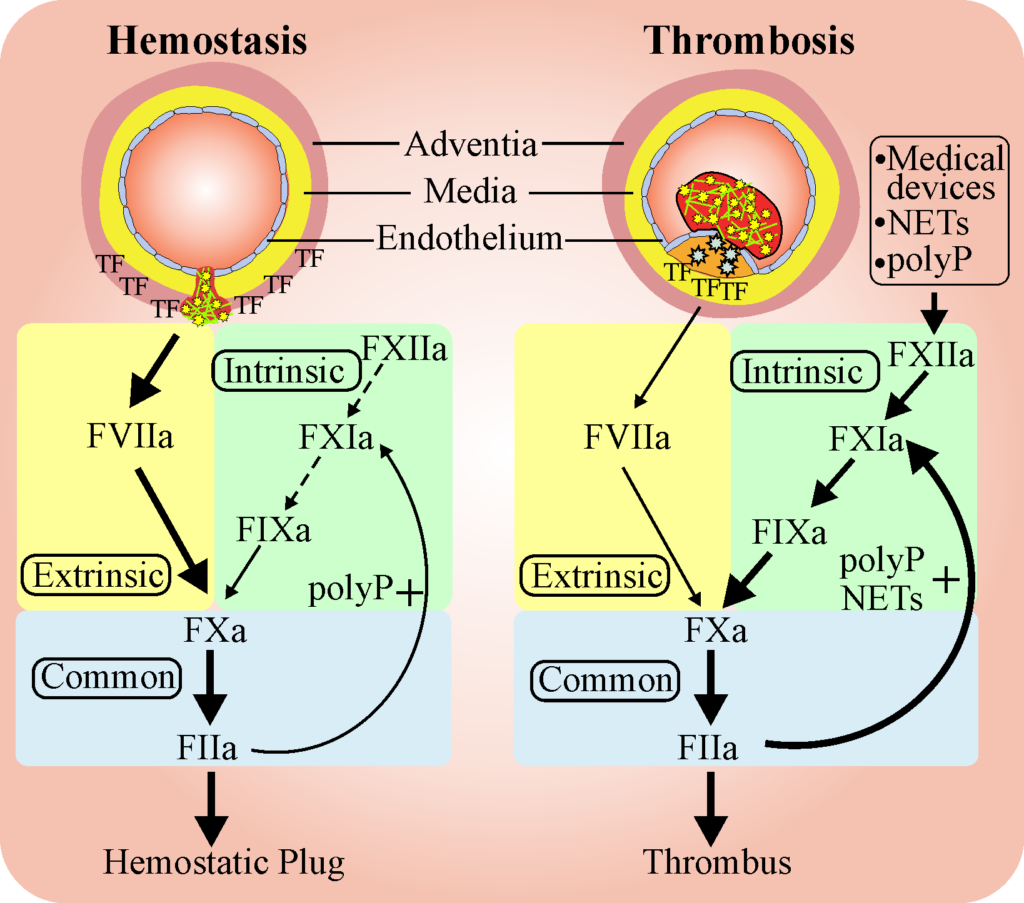
Image shows basic processes of hemostasis and thrombosis. Hemostasis serves to heal wounds in blood vessels and involves a series of reactions that seal the site of injury. Thse steps involve sequential activation of clotting factors (F). Thrombosis represents an over-active hemostasis response due to the presence of injured tissue that releases activating agents such as tissue factor (TF), polyphosphate (PP), or neutrophil extracellular traps (NETS), or due to the presence of medical devices.
Fredenburgh JC, Weitz JI. News at XI: moving beyond factor Xa inhibitors. J Thromb Haemost. 2023 Jul;21(7):1692-1702. doi: 10.1016/j.jtha.2023.04.021.
The Weitz Lab focuses on the regulation of hemostasis, the balance between the processes that promote clot formation (coagulation) and those that promote clot degradation (fibrinolysis). Normally, these systems are balanced such that blood is maintained in a fluid state and flows freely through the vasculature. Numerous regulatory mechanisms maintain this balance, however, dysregulation in either process can cause excessive clotting, which leads to thrombosis or excessive fibrinolysis, which can lead to bleeding.
Thrombosis, which involves abnormal blood clot formation in arteries or veins, is responsible for one in four deaths in Canada and worldwide. Blood clot formation in arteries blocks blood flow and is responsible for heart attack and stroke. Blood clot formation in veins is the underlying cause of deep vein thrombosis. Clots in the deep veins of the leg can break off and travel to the lungs where they plug lung arteries and produce a pulmonary embolism, which can be fatal. A major goal of the Weitz research program is to reduce disability and death caused by thrombosis.
Anticoagulants or “blood thinners” are drugs that attenuate blood clot formation. Anticoagulants are a mainstay for the prevention and treatment of clots in arteries and veins. By attenuating clot formation, the currently available anticoagulants attenuate thrombosis (“bad clots”) by preventing clot formation in arteries or veins. However, they can also cause bleeding by attenuating the formation of clots that plug holes in the blood vessels at sites of injury (“good clots”). A major focus of the Weitz Lab is on the development of safer anticoagulants that attenuate bad clot formation (i.e., thrombosis) without disrupting good clot formation (i.e., hemostasis). Anticoagulants that target factor XI or factor XII are examples of this new class of agents that may be safer than currently available anticoagulants. Studies are underway to better understand how these new agents work and why they are safer than the currently available anticoagulants are underway.
Coagulation can be triggered by damage to or activation of the cells lining the blood vessels, sluggish blood flow,or hypercoagulability of the blood. Medical devices such as catheters, stents or mechanical heart valves can also induce clot formation. The Weitz Lab is investigating these mechanisms. We have identified a protein in the circulation that down regulates clotting induced by medical devices or by clot-promoting substances released from activated blood cells. Studies are underway to determine how this protein shuts down clotting and exploiting this information to develop novel anticoagulants.
On the medical device side, the Weitz Lab is working with bioengineers at McMaster to develop novel coatings that repel protein and blood cell deposition and prevent clotting on the devices. These techniques are being used to design clot resistant catheters, grafts and heart valves.
Finally, the Weitz Lab is investigating a novel approach to attenuating thrombosis by enhancing clot degradation. This can be accomplished by inhibiting the natural blockers of clot breakdown.
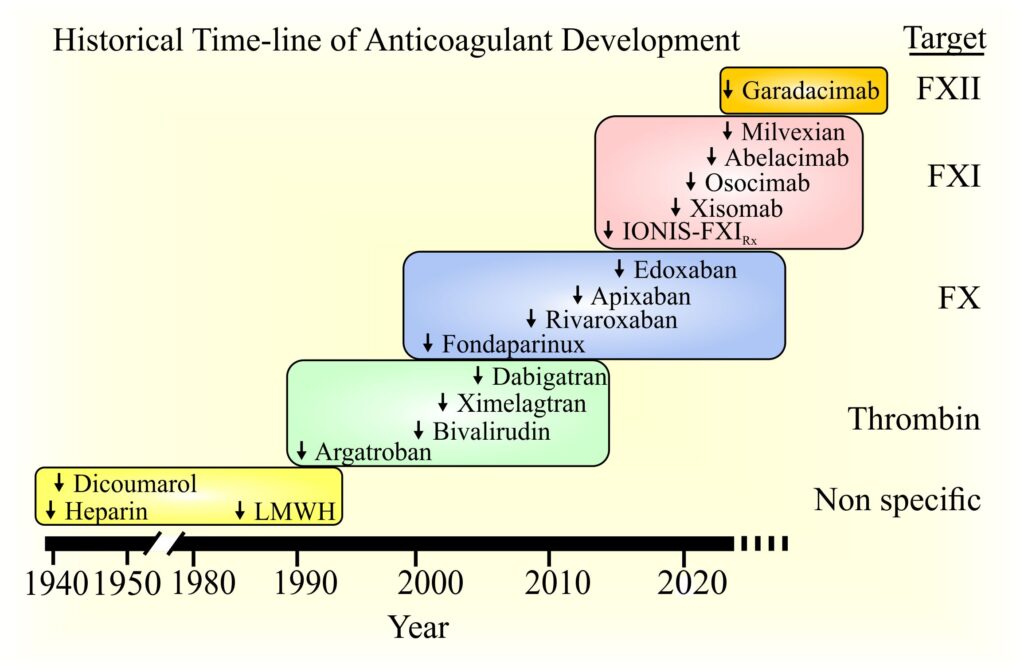
Image shows the time-line of development of anticoagulants.
Initial agents were non-specific and targeted multiple factors (F). But with continued research and experience, novel, direct-acting agents have been developed. Agents directed at thrombin and FXa are approved for clinical use, whereas those targeting FXI and FXII are in clinical trials.
Who We Are
The lab is directed by Dr. Jeffrey Weitz at the Thrombosis and Atherosclerosis Research Institute (TaARI), a joint venture between McMaster University and Hamilton Health Sciences. Dr. Weitz is a hematologist and a clinician-scientist. He is a professor in the Departments of Medicine and Biochemistry and Biomedical Sciences and he is the past president of the International Society on Thrombosis and Haemostasis. The lab is located at the David Braley Research Institute at the Hamilton General Hospital site. The research group is comprised of research associates, undergraduate students, graduate students and postdoctoral fellows.
Information Box Group
Projects
- How medical devices promote clotting and the role of anticoagulants to prevent this
- The role of histidine rich glycoprotein in hemostasis, as it points to unique regulatory processes that may yield novel anticoagulant agents
- How polyphosphate, a polymer abundant in platelets, promotes coagulation and inhibits fibrinolysis
- The mechanism of action of preclinical anticoagulant and thrombolytic drugs
- Identifying sites of interaction of coagulation proteins for structure-function studies
Techniques Used
- Visual and fluorescent spectroscopy
- Enzyme and inhibition kinetics
- Preparative and analytical chromatography
- Surface plasmon resonance
- Clotting assays
- Confocal fluorescent microscopy
- Cell sorting
- Cell culture
- Recombinant DNA
- Isolation of plasma and recombinant proteins
Spacefilling model of thrombin (silver) bound to thrombomodulin (blue sticks) with the active site (red) occupied by an inhibitor (black sticks). Thrombomodulin is a key regulator of thrombin activity, converting it from a procoagulant to an anticoagulant enzyme.
Past Student Career Achievement
- Academic faculty
- Medical doctor
- Dental surgeon
- Business administration
- Clinical chemist
- Pharmaceutical industry
Selected Publications
Recent Bibliography
Current Gaps in the Provision of Safe and Effective Anticoagulation in Atrial Fibrillation and the Potential for Factor XI-Directed Therapeutics.
Goodman SG, Roy D, Pollack CV Jr, Leblanc K, Kwaku KF, Barnes GD, Bonaca MP, True Hills M, Campello E, Fanikos J, Connors JM, Weitz JI.
Crit Pathw Cardiol. 2024 Jun 1;23(2):47-57. doi: 10.1097/HPC.0000000000000351
Histidine-Rich Glycoprotein Modulates the Toxic Effects of High-Dose Polyphosphate in Mice.
Malik RA, Zhou J, Fredenburgh JC, Crosby J, Revenko AS, Healey JS, Weitz JI.
Arterioscler Thromb Vasc Biol. 2024 Jul;44(7):1658-1670. doi: 10.1161/ATVBAHA.124.320899
Human platelets contain a pool of free zinc in dense granules.
Kahr WHA, Henderson SJ, Pluthero FG, Heijnen HFG, Vaezzadeh N, Stafford AR, Fredenburgh JC, Weitz JI.
Res Pract Thromb Haemost. 2024 Feb 15;8(2):102352. doi: 10.1016/j.rpth.2024.102352.
Anticoagulation with osocimab in patients with kidney failure undergoing hemodialysis: a randomized phase 2 trial.
Weitz JI, Tankó LB, Floege J, Fox KAA, Bhatt DL, Thadhani R, Hung J, Pap ÁF, Kubitza D, Winkelmayer WC; CONVERT Investigators.
Nat Med. 2024 Feb;30(2):435-442. doi: 10.1038/s41591-023-02794-7
Histidine-rich glycoprotein attenuates catheter thrombosis.
Malik RA, Liao P, Zhou J, Hussain R, Fredenburgh JC, Hettrick L, Revenko AS, Weitz JI.
Blood Adv. 2023 Sep 26;7(18):5651-5660. doi: 10.1182/bloodadvances.2022009236.
Allosteric modulation of exosite 1 attenuates polyphosphate-catalyzed activation of factor XI by thrombin.
Yin R, Patel V, Malik RA, Fredenburgh JC, Weitz JI.
J Thromb Haemost. 2023 Jan;21(1):83-93. doi: 10.1016/j.jtha.2022.10.001.
Rivaroxaban and apixaban are less effective than enoxaparin for the prevention of catheter-induced clotting in vitro.
Guan Z, Wang R, Hussain RH, Fredenburgh JC, Jaffer IH, Weitz JI.
J Thromb Haemost. 2023 Jan;21(1):76-82. doi: 10.1016/j.jtha.2022.10.020.
Identification of the histidine-rich glycoprotein domains responsible for contact pathway inhibition.
Truong TK, Malik RA, Yao X, Fredenburgh JC, Stafford AR, Madarati HM, Kretz CA, Weitz JI.
J Thromb Haemost. 2022 Apr;20(4):821-832. doi: 10.1111/jth.15631.
Producing Fluorine- and Lubricant-Free Flexible Pathogen- and Blood-Repellent Surfaces Using Polysiloxane-Based Hierarchical Structures.
Ladouceur L, Shakeri A, Khan S, Rincon AR, Kasapgil E, Weitz JI, Soleymani L, Didar TF.
ACS Appl Mater Interfaces. 2022 Jan 26;14(3):3864-3874. doi: 10.1021/acsami.1c21672.
Milvexian for the Prevention of Venous Thromboembolism.
Weitz JI, Strony J, Ageno W, Gailani D, Hylek EM, Lassen MR, Mahaffey KW, Notani RS, Roberts R, Segers A, Raskob GE; AXIOMATIC-TKR Investigators.
N Engl J Med. 2021 Dec 2;385(23):2161-2172. doi: 10.1056/NEJMoa2113194.
Polyphosphate-induced thrombosis in mice is factor XII dependent and is attenuated by histidine-rich glycoprotein.
Malik RA, Zhou J, Fredenburgh JC, Truong TK, Crosby JR, Revenko AS, Weitz JI.
Blood Adv. 2021 Sep 28;5(18):3540-3551. doi: 10.1182/bloodadvances.2021004567.
Abelacimab for Prevention of Venous Thromboembolism.
Verhamme P, Yi BA, Segers A, Salter J, Bloomfield D, Büller HR, Raskob GE, Weitz JI; ANT-005 TKA Investigators.
N Engl J Med. 2021 Aug 12;385(7):609-617. doi: 10.1056/NEJMoa2105872.
Rivaroxaban and Dabigatran for Suppression of Mechanical Heart Valve-Induced Thrombin Generation.
Jaffer IH, Fredenburgh JC, Stafford A, Whitlock RP, Weitz JI.
Ann Thorac Surg. 2020 Aug;110(2):582-590. doi: 10.1016/j.athoracsur.2019.10.091.
Exosite 2-Directed Ligands Attenuate Protein C Activation by the Thrombin-Thrombomodulin Complex.
Chen K, Stafford AR, Wu C, Yeh CH, Kim PY, Fredenburgh JC, Weitz JI.
Biochemistry. 2017 Jun 20;56(24):3119-3128. doi: 10.1021/acs.biochem.7b00250.
*Bold signifies first author trainee
Reviews
Global Health Burden of Venous Thromboembolism.
Wendelboe A, Weitz JI.
Arterioscler Thromb Vasc Biol. 2024 May;44(5):1007-1011. doi: 10.1161/ATVBAHA.124.320151
Arterial Thrombosis: Present and Future
Jeffrey I Weitz
Circulation. 2024 Sep 17;150(12):905-907. doi: 10.1161/CIRCULATIONAHA.124.070541
News at XI: moving beyond factor Xa inhibitors.
Fredenburgh JC, Weitz JI.
J Thromb Haemost. 2023 Jul;21(7):1692-1702. doi: 10.1016/j.jtha.2023.04.021
Reversal agents for current and forthcoming direct oral anticoagulants.
van Es N, De Caterina R, Weitz JI.
Eur Heart J. 2023 May 21;44(20):1795-1806. doi: 10.1093/eurheartj/ehad123.
What Is the Future of Factor XI Inhibitors?
Weitz JI, Eikelboom JW.
Circulation. 2022 Dec 20;146(25):1899-1902. doi: 10.1161/CIRCULATIONAHA.122.061132
State-of-the-Art Mini Review: Dual-Pathway Inhibition to Reduce Arterial and Venous Thromboembolism.
Goldin M, Koulas I, Weitz JI, Spyropoulos AC.
Thromb Haemost. 2022 Aug;122(8):1279-1287. doi: 10.1055/a-1778-1083.
New anticoagulants: Moving beyond the direct oral anticoagulants.
Fredenburgh JC, Weitz JI.
J Thromb Haemost. 2021 Jan;19(1):20-29. doi: 10.1111/jth.15126.
Commentaries
Warfarin faring better: vitamin K antagonists beat rivaroxaban and apixaban in the INVICTUS and PROACT Xa trials.
Eikelboom JW, Weitz JI.
J Thromb Haemost. 2023 Nov;21(11):3067-3071. doi: 10.1016/j.jtha.2023.06.036
Ciraparantag as a potential universal anticoagulant reversal agent.
Chan NC, Weitz JI.
Eur Heart J. 2022 Mar 7;43(10):993-995. doi: 10.1093/eurheartj/ehab706.
International Society on Thrombosis and Haemostasis: Present and future.
Weitz JI, Peyvandi F.
J Thromb Haemost. 2021 Jul;19(7):1599-1601. doi: 10.1111/jth.15325.
Platelet polyphosphate: the long and the short of it.
Weitz JI, Fredenburgh JC.
Blood. 2017 Mar 23;129(12):1574-1575. doi: 10.1182/blood-2017-01-761593.
A PoTENtial Antidote: A Prohemostatic Factor Xa Variant for Reversal of Direct Oral Anticoagulants.
Fredenburgh JC, Weitz JI.
Circ Res. 2016 Nov 11;119(11):1157-1160. doi: 10.1161/CIRCRESAHA.116.309820.
The real decoy: an antidote for factor Xa-directed anticoagulants.
Yeh CH, Fredenburgh JC, Weitz JI.
Circ Res. 2013 Sep 27;113(8):954-7. doi: 10.1161/CIRCRESAHA.113.302297.








